The global healthcare landscape has undergone a monumental shift as we navigate through 2026. We have moved past the era of crisis-driven procurement and entered a phase of sophisticated, endemic disease management. For hospital administrators, lab managers, and procurement officers, this evolution changes the criteria for selecting a virus sampling kit supplier. It is no longer enough to find a vendor that simply has stock in a warehouse. In 2026, clinical accuracy, regulatory compliance, and technological integration define the leaders in the medical consumable space.
Precision in diagnostics begins at the point of collection. If the initial sample degrades because of a low-quality tube or unstable media, even the most advanced laboratory equipment cannot provide a reliable result. This reality places immense pressure on the choice of your virus sampling kit supplier. You are not just buying plastic and liquid; you are investing in the integrity of your diagnostic data. As infectious diseases continue to evolve, the tools we use to capture them must meet higher standards than ever before.
In this exhaustive guide, we will analyze the critical factors that define a world-class virus sampling kit supplier in 2026. We will explore everything from the chemical stability of transport media to the digital transparency of the modern supply chain. By the end of this article, you will have a clear framework for evaluating partners who can support your laboratory’s mission of diagnostic excellence.
Table of Contents
- 1 The Regulatory Benchmark: Beyond Basic Certifications
- 2 Technical Integrity: The Chemistry of Preservation
- 3 Supply Chain Resilience and AI Integration
- 4 The Shift Toward Multiplex and Syndromic Testing
- 5 Environmental Responsibility and Sustainable Procurement
- 6 Comparing Global Manufacturers: A Strategic Approach
- 7 The Importance of Communication and After-Sales Support
- 8 Why Siny Medical is Your Ideal Partner in 2026
- 9 FAQs
The Regulatory Benchmark: Beyond Basic Certifications
One of the most significant changes in 2026 is the tightening of regulatory frameworks across the globe. As of February 2, 2026, the FDA’s Quality Management System Regulation (QMSR) has fully aligned with ISO 13485. This means that a virus sampling kit supplier must demonstrate a level of quality control that was previously only expected in high-end medical device manufacturing. When you evaluate a potential partner, you must look for proactive compliance rather than reactive certification.
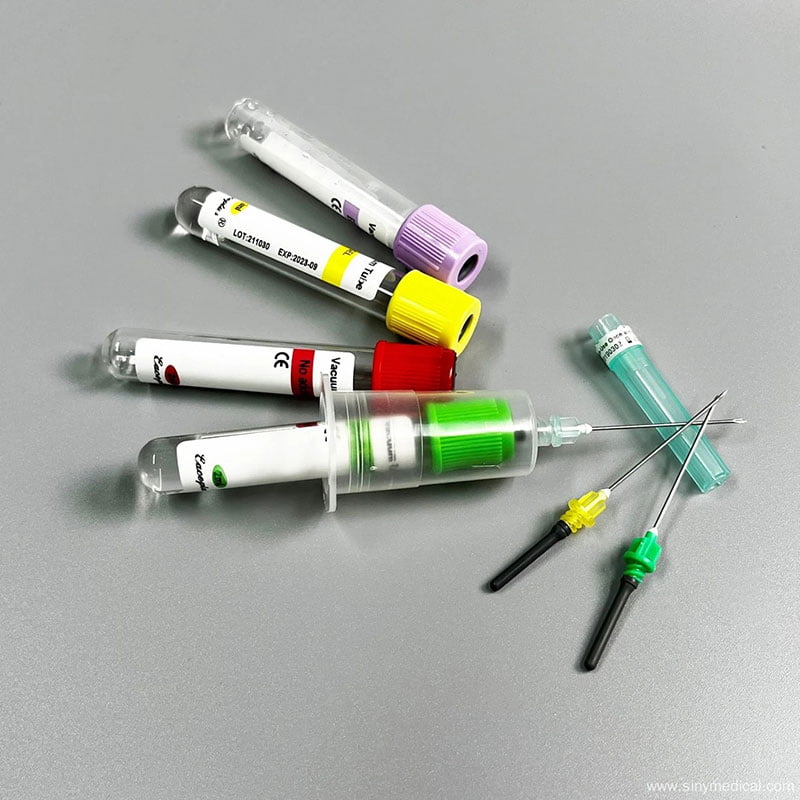
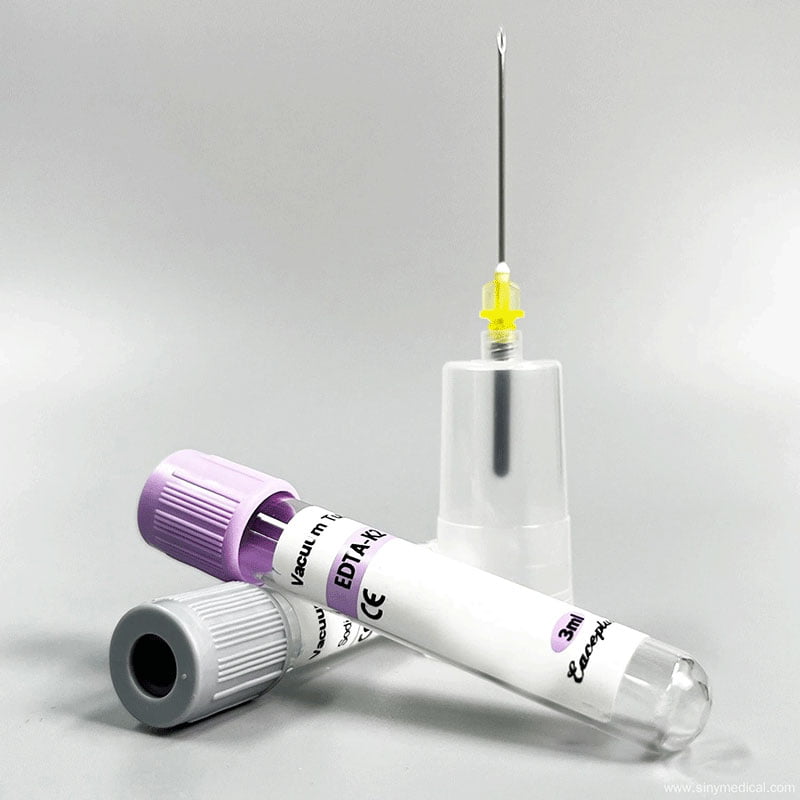
A reliable virus sampling kit supplier should provide transparent access to their audit trails. They must prove that every batch of disposable product virus sampling tube swabs is manufactured in a controlled cleanroom environment. This prevents cross-contamination and ensures that the genetic material collected is the patient’s, not a contaminant from the factory floor. According to the Wikipedia entry on viruses, these biological entities are incredibly small and sensitive. Even microscopic amounts of foreign DNA or RNA in a sampling kit can trigger a false positive in a sensitive PCR test.
Furthermore, you should check if the supplier stays updated with the latest international standards. The transition to the endemic management of viruses like SARS-CoV-2 and Influenza has led to more rigorous stability testing requirements. A supplier that still relies on 2021-era documentation is likely falling behind. You need a partner like Siny Medical, which consistently updates its manufacturing protocols to exceed current regulatory expectations.
Technical Integrity: The Chemistry of Preservation
In 2026, the complexity of viral transport media (VTM) has advanced significantly. We are seeing a move away from “one-size-fits-all” solutions toward specialized media designed for multiplex testing. A top-tier virus sampling kit supplier must offer media that can preserve the integrity of multiple pathogens simultaneously. Whether you are testing for respiratory syncytial virus (RSV), various flu strains, or emerging variants, the chemical buffer in the tube must keep the viral load stable.
Stability is the most important factor here. In the past, many transport media required strict cold-chain management. Today, a leading virus sampling kit supplier provides media that remains stable at room temperature for extended periods. This innovation reduces the logistical burden on clinics and field testing sites. If you want to understand the physics and chemistry behind these improvements, you can read our detailed post on how disposable virus sampling tubes work.
The physical engineering of the tube itself is equally vital. A high-quality exporter virus sampling tube should be made of medical-grade polypropylene with a reinforced screw cap. In 2026, leak-proof technology is a mandatory requirement. If a tube leaks during transit, it creates a biohazard risk for lab personnel and invalidates the patient’s sample. You should look for suppliers who utilize internal O-rings or specialized threading designs to ensure a 100% airtight seal under varying pressure conditions, such as those found in air transport.
Supply Chain Resilience and AI Integration
The supply chain disruptions of the early 2020s taught the medical world a hard lesson. In 2026, a virus sampling kit supplier is judged by their ability to maintain stock levels through intelligent forecasting. Leading manufacturers now use AI-driven inventory management systems to predict surges in demand based on epidemiological data. This ensures that when a seasonal outbreak hits, your laboratory does not face a shortage of essential supplies.
Visibility is the new standard. A modern virus sampling kit supplier offers digital transparency, allowing you to track your orders in real-time. You should be able to see exactly where your shipment is and receive automated alerts if there are any delays. This level of communication is critical for laboratories that run high-volume testing schedules and cannot afford even a single day of downtime.
Many laboratories are now looking directly at the source to ensure supply chain stability. By working with a direct manufacturer rather than a middleman, you can often secure better pricing and more reliable lead times. You can see the scale of modern production by visiting the Siny Medical YouTube channel, which showcases the automated manufacturing processes that drive the 2026 supply chain. Additionally, reputable suppliers often list their credentials on international platforms like Made-in-China to facilitate global trust.
The Shift Toward Multiplex and Syndromic Testing
As we move deeper into 2026, laboratories are shifting away from single-pathogen tests. Instead, they are adopting syndromic testing panels that can identify multiple viruses from a single swab. This change places new demands on the virus sampling kit supplier. The kits must be designed to release as much biological material as possible into the transport medium to ensure there is enough genetic matter for multiple assays.
Flocked swabs have become the industry standard for this reason. Unlike traditional fiber-wrapped swabs, flocked swabs utilize perpendicular nylon fibers that act like a soft brush. This design improves specimen collection and, more importantly, ensures that the specimen is completely “flushed” into the liquid media. A knowledgeable virus sampling kit supplier will help you choose the right swab type for your specific testing equipment. You can find more information about these specialized tools in the ultimate guide to disposable virus sampling tubes.
Environmental Responsibility and Sustainable Procurement
In 2026, sustainability has moved from a “nice-to-have” feature to a core procurement criterion. The medical industry produces a massive amount of plastic waste, and laboratories are under increasing pressure to reduce their environmental footprint. A forward-thinking virus sampling kit supplier is actively looking for ways to minimize waste. This might include using thinner but stronger medical-grade plastics, reducing secondary packaging, or implementing recycling programs for non-biohazardous components.
While the primary goal of a virus sampling tube is safety and sterility, the packaging it arrives in can be optimized. Suppliers who use biodegradable materials for their outer cartons or offer bulk packaging options help laboratories meet their ESG (Environmental, Social, and Governance) targets. When interviewing a potential virus sampling kit supplier, ask about their sustainability roadmap. A company that cares about the future of the planet is likely to care about the quality of the products they provide to your patients.
Comparing Global Manufacturers: A Strategic Approach
Choosing the right partner involves more than just comparing a price list. You need to look at the total value of the relationship. Below is a comparison of what characterizes a high-value virus sampling kit supplier versus a standard vendor in the 2026 market.
| Feature | Standard Vendor | High-Value Supplier (2026 Standard) |
| Regulatory Status | Basic CE/ISO marks | FDA QMSR & ISO 13485:2026 Compliant |
| Media Stability | Requires cold-chain (2-8°C) | Room temperature stable (up to 12 months) |
| Supply Chain | Reactive (Stock-out prone) | Proactive (AI-driven forecasting) |
| Technical Support | Minimal / Sales focused | Clinical & Laboratory guidance provided |
| Product Range | Limited to 1-2 types | Full range of virus sampling tube categories |
| Transparency | Hidden manufacturing | Open factory audits and video verification |
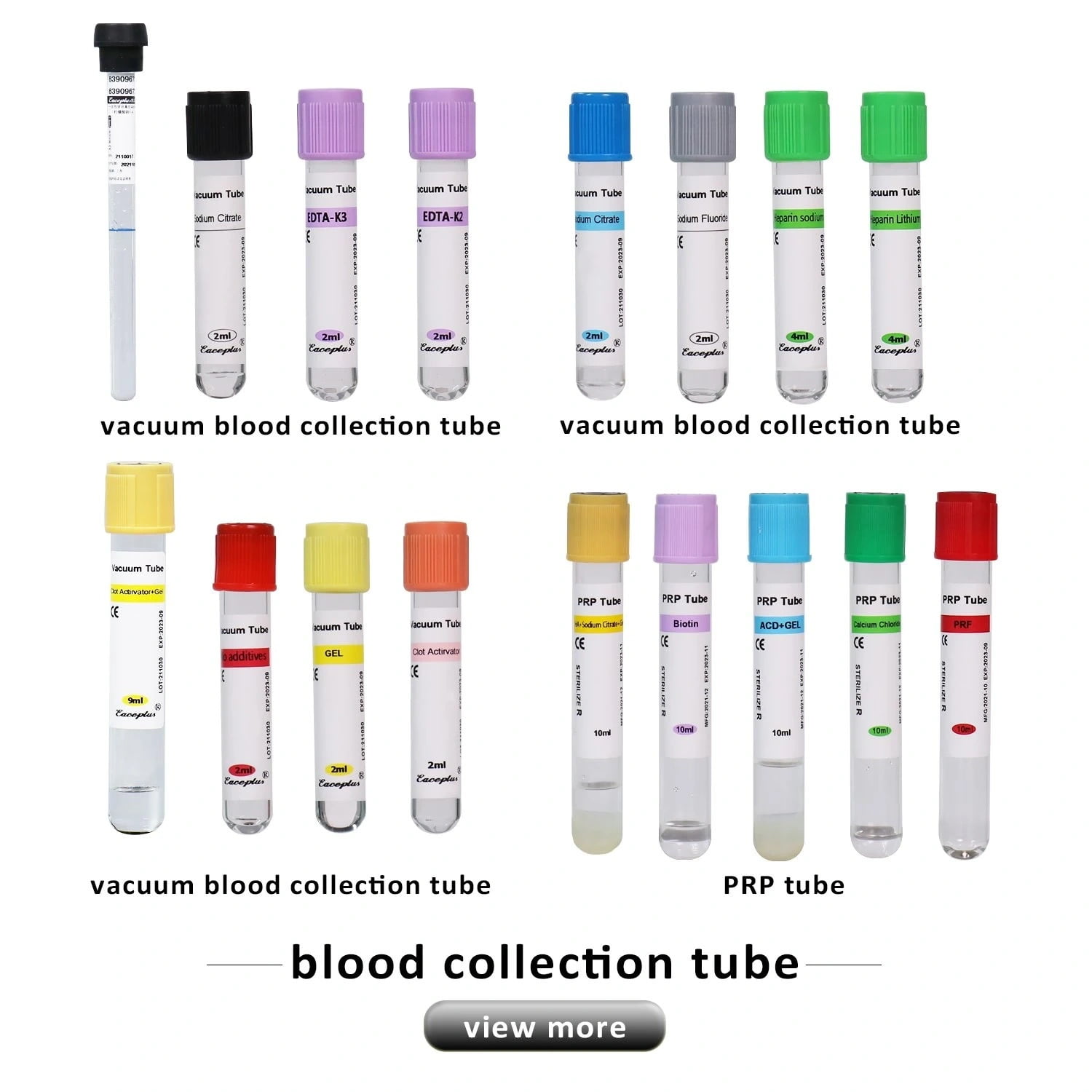
A high-value virus sampling kit supplier acts as an extension of your laboratory team. They provide you with a virus sampling tube usage guide and technical data sheets that help you optimize your internal workflows. They also offer a diversified product portfolio, including other essential items like blood collection tubes, which allows you to consolidate your medical consumable procurement.
The Importance of Communication and After-Sales Support
In the medical field, questions and issues can arise at any hour. A virus sampling kit supplier that disappears once the invoice is paid is a liability. In 2026, you should prioritize suppliers who offer robust after-sales support. This includes providing replacements for any damaged goods, technical assistance for new lab equipment integration, and timely communication regarding any regulatory changes.
Responsiveness is a key indicator of reliability. Before you sign a long-term contract, test the supplier’s communication. Do they answer technical questions accurately? Is there a dedicated account manager who understands your laboratory’s specific needs? A company that values its reputation will make it easy for you to contact Siny Medical and get the answers you need immediately.
Why Siny Medical is Your Ideal Partner in 2026
As we have explored, the requirements for a virus sampling kit supplier in 2026 are more demanding than ever. Siny Medical has spent years preparing for this shift by investing in state-of-the-art automation, rigorous quality control systems, and a globally resilient supply chain. We understand that our products are the first line of defense in global health, and we take that responsibility seriously.
Our commitment to innovation ensures that our kits are compatible with the latest multiplex testing platforms. We provide room-temperature stable media that simplifies your logistics and high-quality flocked swabs that maximize sample yield. Whether you are running a small clinical lab or a large-scale hospital network, we have the capacity and the expertise to support your operations.
By choosing Siny Medical as your virus sampling kit supplier, you are choosing diagnostic accuracy, regulatory peace of mind, and a partnership built on transparency. We don’t just sell products; we provide the foundation for healthy communities.
FAQs
1. What certifications should a virus sampling kit supplier have in 2026?
In 2026, a reputable virus sampling kit supplier should hold ISO 13485:2016 certification and demonstrate full compliance with the FDA’s Quality Management System Regulation (QMSR). These standards ensure that the manufacturing process is consistent, sterile, and well-documented. Additionally, look for CE marking if you are operating in the European market, as this indicates the product meets high safety and health requirements.
2. How does a virus sampling kit supplier ensure the stability of the transport media?
A top-tier virus sampling kit supplier uses advanced chemical buffering agents to maintain a stable pH environment for the viral specimens. In 2026, many suppliers offer “non-inactivating” media that keeps the virus viable for culture and “inactivating” media that lyses the virus for safer handling while preserving RNA/DNA. High-quality suppliers also conduct rigorous accelerated aging tests to ensure the media remains effective throughout its entire shelf life.
3. Why is AI integration important for a virus sampling kit supplier?
AI integration allows a virus sampling kit supplier to monitor global health trends and predict potential demand spikes. This prevents the “bullwhip effect” in the supply chain where laboratories face sudden shortages. Furthermore, AI helps in the manufacturing process by utilizing computer vision to detect microscopic defects in tubes or swabs, ensuring that every kit that reaches your lab is of perfect quality.
4. Can a virus sampling kit supplier provide customized kits?
Yes, a flexible virus sampling kit supplier like Siny Medical can often customize kits to meet specific laboratory protocols. This might include changing the volume of the transport media, providing different swab sizes (nasopharyngeal vs. oropharyngeal), or using specialized labeling for automated lab systems. Customization is particularly important for large-scale clinical trials or research projects with unique sampling requirements.