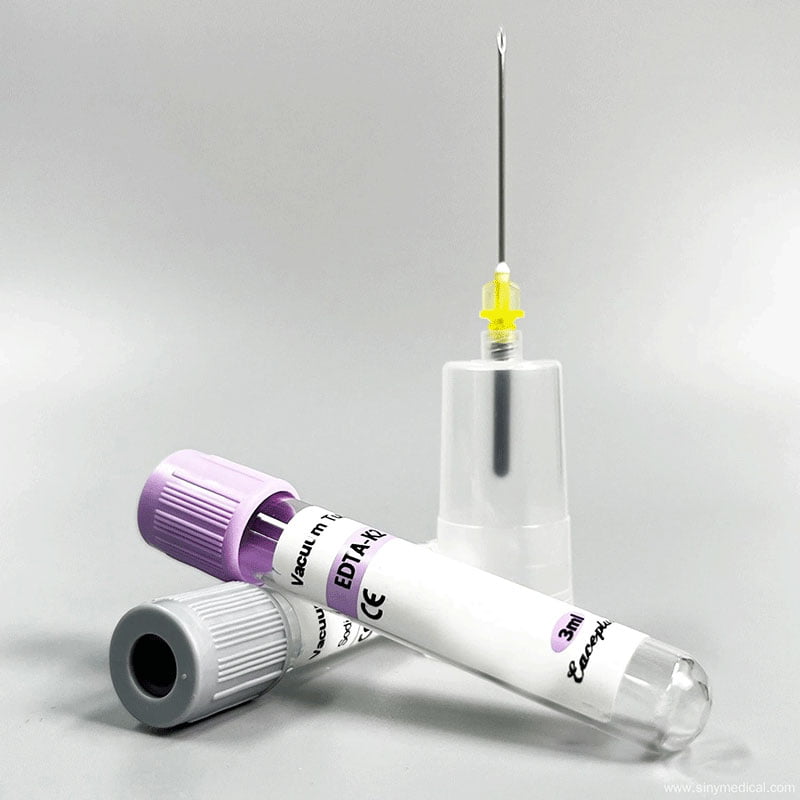
In the world of modern medical diagnostics, the colorful array of blood collection tubes serves as the first, most critical gatekeeper for accurate results. Among them, the tube with the distinctive purple top—the EDTA tube—is arguably the most ubiquitous. It’s a cornerstone of everything from routine annual physicals to complex disease diagnostics. But how well do we truly understand this workhorse of the lab? What are the real-world differences between the different EDTA tubes available, such as those containing K2, K3, or the older Na2? Does this small variation truly impact patient results?
As your trusted partner in medical supplies, this guide will take you on a comprehensive journey into the world of EDTA anticoagulant tubes. We’ll break down the science, compare the critical differences between various types, and outline the best practices for clinical application, providing you with a definitive, practical resource.
Table of Contents
What is EDTA, and How Does It Prevent Blood from Clotting?
The body’s natural response to injury is a complex cascade of events leading to blood coagulation. Central to this process is the calcium ion (Ca²⁺), which acts as a vital catalyst and messenger, enabling clotting factors to work correctly.
EDTA, an acronym for Ethylenediaminetetraacetic Acid, is a powerful chelating agent. In simple terms, it acts like a “calcium magnet.” When blood enters an EDTA tube, the EDTA immediately and irreversibly “grips” onto the free calcium ions in the sample. This process preserves the blood in its liquid state, perfectly maintaining the original count, morphology, and integrity of blood cells, which is essential for hematological analysis.
K2 vs. K3 vs. Na2: Comparing the Different EDTA Tubes
This is the most crucial discussion when selecting the right purple top tube. While all are salts of EDTA, their physical properties and subtle effects on test samples differ significantly.
EDTA K2 – Dipotassium EDTA
- Form: Applied to the inner wall of the tube as a fine, spray-dried coating.
- Advantages: As a dry additive, EDTA K2 does not dilute the blood sample, thus preserving the original concentration of blood components. The International Council for Standardization in Haematology (ICSH) officially recommends EDTA K2 as the anticoagulant of choice for blood cell counting and sizing. This is because it has the least impact on the volume of red and white blood cells, ensuring a more accurate reflection of the patient’s true cellular state.
EDTA K3 – Tripotassium EDTA
- Form: Historically, K3 was often used as a liquid additive.
- Potential Impact: The liquid form inevitably causes a slight 1-2% dilution of the blood sample. While minor, this dilution can lead to small but significant inaccuracies in highly sensitive tests like the Packed Cell Volume (PCV) or Hematocrit (HCT). Furthermore, some studies suggest that higher concentrations of K3 can affect white blood cell sizing on certain hematology analyzers.
EDTA Na2 – Disodium EDTA
- Form: Typically found in crystalline or powder form.
- Key Limitation: Disodium EDTA dissolves in blood more slowly than its potassium salt counterparts. This slower action can lead to incomplete or delayed anticoagulation, increasing the risk of microclot formation, especially if the tube isn’t mixed promptly. These microclots can interfere with cell counters. Due to its inferior solubility and performance, this type of EDTA tube has been largely phased out of modern clinical hematology.
The Verdict: While all three can function as anticoagulants, EDTA K2 stands as the undisputed gold standard for high-quality diagnostics. Its advantages of zero sample dilution and minimal effect on cell morphology make it the superior choice. EDTA K3 is a functional alternative, while EDTA Na2 is now considered obsolete for most applications.
The Scope of EDTA Tubes: What They Can and Cannot Be Used For
Choosing the correct tube is fundamental to laboratory quality control. The applications for EDTA tubes are highly specific.
Recommended For:
- Complete Blood Count (CBC): This is the primary use, including WBC, RBC, and platelet counts, as well as hemoglobin (HGB), Mean Corpuscular Volume (MCV), and other indices.
- Blood Typing: Determining ABO and Rh groups.
- Glycated Hemoglobin (HbA1c): Assessing long-term glycemic control in diabetic patients.
- Reticulocyte Count: Evaluating bone marrow function.
- Molecular Diagnostics: Used for DNA extraction from whole blood for genetic testing.
Do Not Use For:
- Coagulation Studies: Tests like Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) require a light blue top tube containing Sodium Citrate.
- Most Clinical Chemistry Tests: EDTA’s calcium-binding properties make it unsuitable for measuring electrolytes like calcium, potassium, or sodium. It also inhibits the activity of various enzymes, such as Alkaline Phosphatase (ALP). These tests typically require a gold top (SST) or red top (serum) tube.
Precision Starts with Procedure: Best Practices for Using EDTA Tubes
A high-quality tube is only as good as the technique used to draw the sample.
- Follow the Order of Draw: When healthcare professionals require multiple tubes, they should draw the EDTA tube towards the end. This ensures proper sample collection and prevents cross-contamination of additives. The recommended CLSI order of draw is typically: Blood Cultures -> Coagulation (light blue) -> Serum (red/gold) -> EDTA (purple) -> Other anticoagulants. This prevents cross-contamination of additives.
- Ensure Correct Fill Volume: The amount of EDTA in each tube is precisely measured for its stated draw volume. Under-filling or over-filling disrupts the critical blood-to-anticoagulant ratio, which can lead to clotting (under-filled) or cellular distortion (over-filled).
- Mix Immediately and Gently: This is the most critical and often overlooked step. As soon as you fill the tube, gently invert it 8-10 times to ensure the blood thoroughly mixes with the EDTA coating the walls. Do not shake vigorously, as this can damage blood cells (hemolysis).
Wrapping It Up
From the chemistry of calcium chelation to the subtle differences between K2, K3, and Na2 EDTA tubes, we clearly see that not all purple tops are created equal. In fact, the choice of tube can make the difference between accurate results and misleading ones.
For modern diagnostics, K2 EDTA stands as the gold standard, ensuring cell preservation, stable counts, and reliable analyzer performance. Whether it’s a routine CBC, DNA extraction, or HbA1c monitoring, the right EDTA tube safeguards both patient safety and laboratory accuracy.
At Siny Medical, we pride ourselves on delivering high-quality EDTA tubes for blood collection that meet international standards. For bulk orders, custom specifications, or expert guidance, visit our product category page or connect with us through our Made-in-China profile.
And don’t forget to check out Siny Medical on YouTube for product demos and lab insights.
FAQs
Why are EDTA tubes always purple?
Because purple (or lavender) is the universal color-code for EDTA, helping labs instantly recognize and avoid mix-ups.
What happens if I forget to mix the tube after collection?
Microclots may form, clogging hematology analyzers and producing falsely low counts—often requiring a redraw.
Can I use a partially filled EDTA tube?
No. An under-filled tube alters the blood-to-EDTA ratio, causing falsely low hematocrit and MCV values.
Can I visually tell if it’s K2 or K3 EDTA?
Not by looking at the coating. Always check the manufacturer’s label.
For more guidance, contact Siny Medical directly.